Heat Capacity of Calorimeter
7 Q t c s m s C Δ T 8 Q t Δ T c s. What your book is probably asking is for what is called the calorimeter constant.

Specific Heat Of A Metal Lab Youtube Science Chemistry Chemistry Class Chemistry Labs
The heat capacity of 1 gram of a substance is called its specific heat capacity or specific heat while the heat capacity of 1 mole of a substance is called its molar heat capacity.

. This is given in units of puJcirc C notice that it does not include mass. Heat capacity is the amount of heat required to change the temperature of a given amount of matter by 1C. The manufacturer of the bomb calorimeter determined the heat capacity of the calorimeter to be 114 kJC.
Free easy returns on millions of items. The heat capacity C of the calorimeter can be determined in advance by mixing experiments see next section. So we just need to convert the grams g part of the units to moles mol.
Use 4184 J g 1 C 1 as the specific. The final temperature at equilibrium is 53. This is the currently selected item.
Calculate the mass of the silver sample. 1 Take 50 ml distilled. Watch and measure the temperature.
A calorimeter constant Cal is a measurement of the heat capacity of a calorimeter. Calorimetry is the process by which the heat of a chemical reaction or the physical changes as well as the heat capacity is measured with the help of a device named a calorimeter. It takes 125 kJ of energy to heat a sample of pure silver from 120C to 152C.
Calculate the heat of combustion per gram of gummy bear. Calculate the heat capacity of the calorimeter in JC. Another common use of a coffee-cup calorimeter is to just use it to determine the heat capacity of another substance - like a metal.
Free shipping on qualified orders. Buy 1 Get 2 Free. 125 kJ 1 1000 J.
Did you report your data to the correct number of significant figures. In brief a calorimeter is an instrument that holds the capacity to measure calorimetryCalorimetry follows the principle of the law of conservation of energy which implies heat loss is equal to heat gainIn a calorimeter two forms of matter. Heat your metal sample up to a known temperature usually with boiling water at 100 C and then drop the warmhot piece of metal into the cup containing your much cooler water.
After mixing 1000 g of water at 585 C with 1000 g of water already in the calorimeter at 228 C the final temperature of the water is 397 C. Well we know the specific heat capacity s is 024 JgC. Ad Browse discover thousands of brands.
Ccal qcal Δtcold 4184 in J g-1 C-1 X m1 m2 in g --------------eq 4 The unit of heat capacity is J C-1. A simple calorimeter consists of a thermometer that is attached to a metal container full of water that is suspended above the combustion chamber. If you wanted to use this whole formula for solving the calorimeters specific heat capacity you would need to know the mass of the calorimeter as well which is not given.
Ad IKA offers a wide range of innovative lab equipment for numerous applications in RD. Read customer reviews find best sellers. The heat capacity of calorimeter Ccal is the quantity of heat absorbed by the calorimeter for every one degree rise in temperature of reaction and can be determined by the following formula.
The temperature started at 215 C and leveled off at 242 C. Calorimeter is a device that is used to measure heat energy transfer or thermal energy transfer from one object to another. Heat capacity and calorimetry.
Follow this example to calculate the heat capacity of a calorimeter. 50 g of water at 23 degrees C is mixed with 50 g at 88 degrees C in the calorimeter. It can be calculated by applying a certain quantity of heat to the calorimeter and then measuring the temperature change Multiplying the change in enthalpy H in joules by the change in temperature T kelvins or degrees Celsius in SI units yields the.
A 088 g gummy bear is burned in a bomb calorimeter. In this example we calculate the heat capacity of a bomb calorimeter using constant volume calorimetry given the change in internal energy for a combustion. In this way the specific heat capacity c s of the liquid can finally be determined relatively accurately on the basis of the emitted heat Q t and the temperature change ΔT.
ΔE C V 3078 kJ088 g 3498 kJg. Temperature of cold water C Temperature of hot water C 20 920 Volume of cold water mL 990 Volume of hot water mL 950 Final. Energy of phase changes.
The amount of heat gained or lost by a sample q can be calculated using the equation q. HEAT CAPACITY OF A CALORIMETER INTRODUCTION LABORATORY SIMULATION A Lab Data - X Check your volume measurement. This video teaches the viewer how to calculate the heat capacity of a coffee cup calorimeter.
A student wishes to determine the heat capacity of a coffee-cup calorimeter.
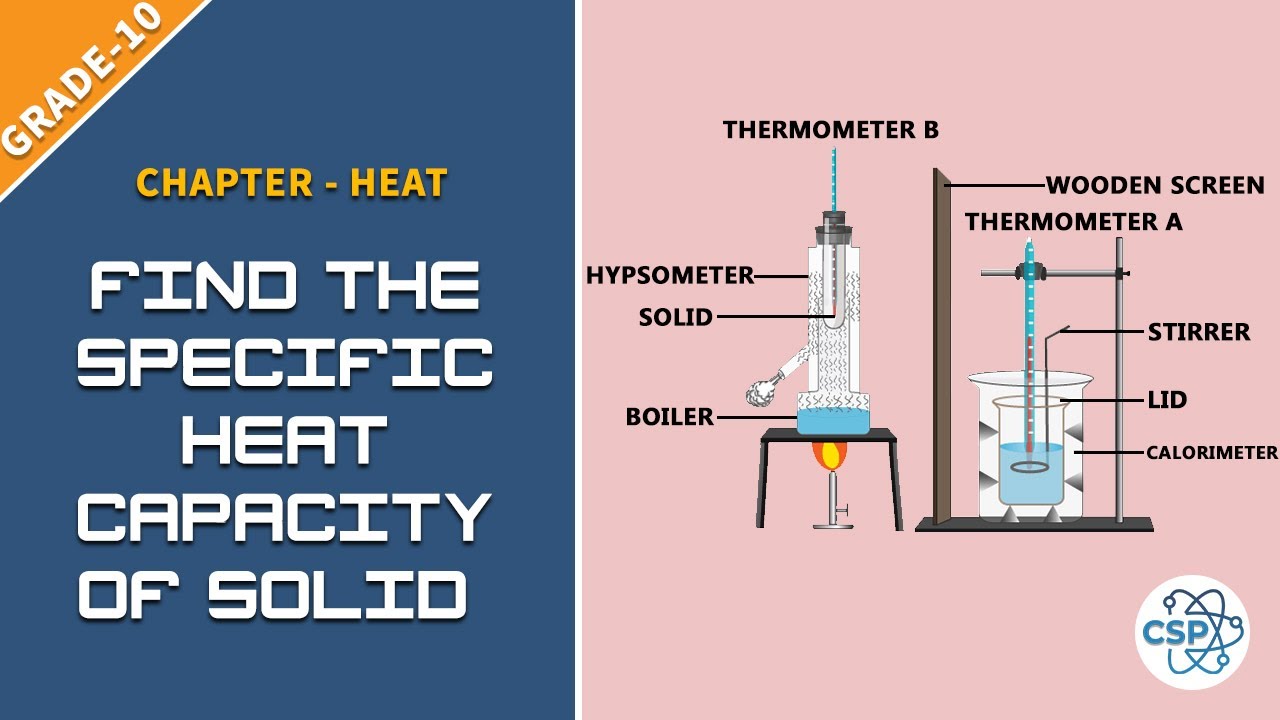
To Find The Specific Heat Capacity Of Solid By Using Method Of Mixtures See Class 10 Physical Properties Heat Science Experiments

Specific Heat Capacity Physics Lessons Science Teaching Resources Science Facts

Pin By Redacted On Chemistry Education Chemistry Education What Is Science Ap Chem

Constant Volume Calorimetry For More Precise Work Than The Coffee Cup Calorimeter The Heat Capacity Of The Entire C Coffee Cups Chemistry Education Chemistry

Comments
Post a Comment